Baseline Data
Baseline Data
Of the 206 patients identified either by International Classification of Diseases, ninth revision, codes for S aureus pneumonia and/or by concomitant blood and respiratory cultures positive for S aureus, 60 met the strict inclusion criteria as assessed for NBSAP. The primary reasons for exclusion from the study included a lack of clinical or radiologic findings supporting a pneumonia diagnosis (n = 46), alternate clinical diagnoses (n = 43), infection that was not nosocomial (n = 26), time correlation between microbiological findings (n = 25), and chart not available (n = 6). Alternative clinical diagnoses potentially causing the infection isolated more than once included the following: endocarditis (n = 12); IV line-related sepsis (n = 16); alternative pathogen most likely caused infection (n = 5); and pelvic abscess (n = 2). Overall, the mean (± SD) age was 55.5 ± 15.0 years, and the median APACHE II score was 20 (range, 3 to 41). Most patients were men (66.7%), and were predominantly African American (56.7%) and white (38.3%). An equal number of patients were admitted to surgery (41.7%) and medicine services (41.7%), and 16.7% of the patients were admitted to the burn unit. Twenty-three patients (38.3%) underwent surgery < 1 month prior to the development of NBSAP. Bilateral infiltrates were evident on chest radiography in 75% of the patients identified. Most patients were in the ICU at time of onset of NBSAP (93.3%), and 83.3% of patients were receiving mechanical ventilation. The median length of stay prior to the onset of NBSAP was 9 days (range, 2 to 81 days).
The origin of the respiratory cultures used for the microbiological determination of pneumonia came from aspirated sputum in 90% of the cases, and from BAL fluid in 10% of cases. Of the 60 patients, 42 patients (70%) were infected with MRSA. Forty-four patients (73.3%) had concomitant organisms (6 in blood only, 7 in blood and respiratory cultures, and 31 in respiratory cultures only). The most common concomitant organisms in the blood were as follows: Enterococcus spp (five cases); Acinetobacter bau-mannii (three cases); Streptococcus viridans (two cases); Klebsiella pneumoniae (one case); Streptococcus pneumoniae (one case); and one cogulase-nega-tive Staphylococcus sp. The most common concomitant respiratory organisms were P aeruginosa (nine cases); A baumannii (eight cases); Klebsiella spp (seven cases); Escherichia coli (six cases); Candida albicans (four cases); Enterobacter spp (three cases); and nine others.
 The clinical and microbiological success rates at the end of treatment were 56.7% and 53.3%, respectively. Thirty-three patients (55.5%) died during hospitalization, and 24 (40.0%) died secondary to NBSAP (ie, IRM). Nine patients died for reasons not attributable to the pneumonia. In seven cases, treatment for NBSAP had stopped at least 2 weeks before the patient died. Withdrawal from support occurred in two patients irrespective of the concomitant pneumonia.
The clinical and microbiological success rates at the end of treatment were 56.7% and 53.3%, respectively. Thirty-three patients (55.5%) died during hospitalization, and 24 (40.0%) died secondary to NBSAP (ie, IRM). Nine patients died for reasons not attributable to the pneumonia. In seven cases, treatment for NBSAP had stopped at least 2 weeks before the patient died. Withdrawal from support occurred in two patients irrespective of the concomitant pneumonia.
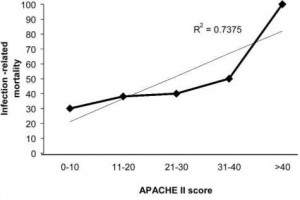
The relationship between APACHE II score and death is shown in Figure 1. A linear relationship existed demonstrating that patients who were more acutely ill at the time of admission to the ICU had a greater mortality rate (R2 = 0.74).
Outcomes of Bacteremic MRSA vs Methicillin-Sensitive S aureus Pneumonia MSSAP
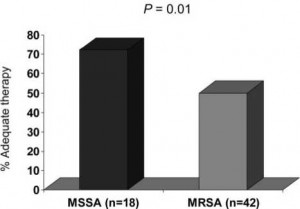
Of the 60 patients, 42 patients (70%) were infected with MRSA. A comparison of clinical features between MRSA and methicillin-sensitive S aureus (MSSA) patients is presented in Table 1. By univariate analysis, MRSA patients were more likely than MSSA patients to have received prior antibiotic treatment and had a longer median length of stay prior to the onset of NBSAP. In addition, a significant difference existed between the two groups with respect to appropriate treatment. In the MSSA group, 72.2% of patients received appropriate therapy within 44.75 h of the onset of infection compared with only 50% of patients in the MRSA subgroup (p = 0.01) [Fig 2]. Furthermore, MSSA pneumonia patients received appropriate treatment in significantly less time than did MRSA pneumonia patients (24 h [range, 8 to 51 h] vs 44 h [range, 1 to 149 h], respectively; p = 0.013). No difference was detected in the duration of therapy between the two groups (MSSA group, 10 days [range, 2.5 to 25.0 days]; MRSA group, 11 days [range, 1.0 to 21.5 days]).
 Of the 18 MSSA patients, 8 patients (44.4%) were empirically treated with vancomycin, 7 patients (38.9%) were empirically treated with a (3-lactam, 1 patient was empirically treated with clindamycin, 1 patient was empirically treated with levofloxacin, and 1 patient died prior to identification of the causative organism and subsequent appropriate Canadian Neighbor Pharmacy antibiotic therapy application. Once sensitivity reports were obtained, 77.8% of the MSSA patients received a (3-lactam agent as the primary antimicrobial treatment. The median length of time needed to switch to optimal therapy for the eight patients who had been empirically treated with vancomycin was 71.5 h (range, 16 to 123 h). Of the 42 MRSA patients, 24 (57.1%) received empiric vancomycin treatment, 8 (19.0%) received (3-lactam agents, 6 (14.3%) received clindamycin, 3 (7.1%) received trimethoprim/sulfamethoxazole, 1 was started on therapy with levofloxacin; and 1 patient died before receiving appropriate antibiotic treatment. Once susceptibility data were available, 40 patients (95.2%) received vancomycin for primary antimicrobial treatment of pneumonia. Two patients were never appropriately treated with antibiotics. Nine patients (50.0%) in the MSSA subgroup were treated with combination therapy compared with seven patients (16.7%) in the MRSA subgroup. The median duration of combination therapy was 3 days in both groups (MSSA group range, 1 to 10 days; MRSA group range, 1 to 8 days). Eleven vancomycin trough levels were collected in a total of nine patients with a median value of 17.4 ^g/mL (range, 9.2 to 26.7 ^g/mL).
Of the 18 MSSA patients, 8 patients (44.4%) were empirically treated with vancomycin, 7 patients (38.9%) were empirically treated with a (3-lactam, 1 patient was empirically treated with clindamycin, 1 patient was empirically treated with levofloxacin, and 1 patient died prior to identification of the causative organism and subsequent appropriate Canadian Neighbor Pharmacy antibiotic therapy application. Once sensitivity reports were obtained, 77.8% of the MSSA patients received a (3-lactam agent as the primary antimicrobial treatment. The median length of time needed to switch to optimal therapy for the eight patients who had been empirically treated with vancomycin was 71.5 h (range, 16 to 123 h). Of the 42 MRSA patients, 24 (57.1%) received empiric vancomycin treatment, 8 (19.0%) received (3-lactam agents, 6 (14.3%) received clindamycin, 3 (7.1%) received trimethoprim/sulfamethoxazole, 1 was started on therapy with levofloxacin; and 1 patient died before receiving appropriate antibiotic treatment. Once susceptibility data were available, 40 patients (95.2%) received vancomycin for primary antimicrobial treatment of pneumonia. Two patients were never appropriately treated with antibiotics. Nine patients (50.0%) in the MSSA subgroup were treated with combination therapy compared with seven patients (16.7%) in the MRSA subgroup. The median duration of combination therapy was 3 days in both groups (MSSA group range, 1 to 10 days; MRSA group range, 1 to 8 days). Eleven vancomycin trough levels were collected in a total of nine patients with a median value of 17.4 ^g/mL (range, 9.2 to 26.7 ^g/mL).
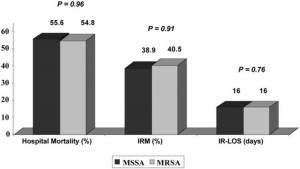
The comparison values of hospital mortality, IRM, and IR-LOS between MSSA and MRSA patients are displayed in Figure 3. No significant differences in these primary end points were observed between the groups. Similarly, no significant differences in hospital mortality, IRM, and IR-LOS were noted for the different empiric antibiotic regimens, stratified by methicillin susceptibility. Multivariate analyses were performed, and the associations between methicillin susceptibility and outcomes were identical to those from the univariate analyses.
 IRM was higher in the empiric vancomycin group than in the empiric (3-lactam group; however, this was not statistically significant. For the 18 patients infected with MSSA, the hospital mortality and IRM rates in the patients receiving empiric (3-lactams were 57.1% and 28.6%, respectively, compared with 62.5% and 50.0%, respectively, in the empiric vancomycin group. For the 42 patients infected with MRSA, the hospital mortality and IRM rates in patients receiving empiric vancomycin were 50.0% and 45.8%, respectively, compared with 62.5% and 25.0%, respectively, in the empiric (3-lactam group. These findings, however, were not statistically significant, and an additional evaluation of this subset revealed that patients who had been empirically treated with (3-lactams tended to be younger (median age, 52 vs 56 years, respectively) and to have lower APACHE II scores (median, 17 vs 21, respectively).
IRM was higher in the empiric vancomycin group than in the empiric (3-lactam group; however, this was not statistically significant. For the 18 patients infected with MSSA, the hospital mortality and IRM rates in the patients receiving empiric (3-lactams were 57.1% and 28.6%, respectively, compared with 62.5% and 50.0%, respectively, in the empiric vancomycin group. For the 42 patients infected with MRSA, the hospital mortality and IRM rates in patients receiving empiric vancomycin were 50.0% and 45.8%, respectively, compared with 62.5% and 25.0%, respectively, in the empiric (3-lactam group. These findings, however, were not statistically significant, and an additional evaluation of this subset revealed that patients who had been empirically treated with (3-lactams tended to be younger (median age, 52 vs 56 years, respectively) and to have lower APACHE II scores (median, 17 vs 21, respectively).
Overall clinical success was achieved in 59.5% of MRSA patients compared with 50.0% of MSSA patients. No differences in clinical or microbiological success were determined at days 3, 7, or 10, or at the end of antimicrobial therapy for any treatment regimen, stratified by methicillin susceptibility. Similarly, because 83.3% of patients were receiving mechanical ventilation at the time of onset, no difference in clinical response was observed in this VAP subset of patients compared with the whole cohort. Of note, the overall clinical cure rate in patients receiving vancomycin was 56.3%; 58.3% in the subset of MRSA patients.
Outcomes in Delayed vs Early Appropriate Therapy
Five patients were excluded in the appropriate therapy analyses secondary to death within 72 h of the onset of infection. Of the remaining 55 patients, 24 patients (43.6%) did not receive appropriate antibiotic treatment within 44.75 h of the onset of infection, and 31 patients (56.4%) received appropriate antibiotic treatment within 44.75 h of the onset of infection. There were no significant differences between the two groups (delayed vs early) with respect to the APACHE II score at time of admission to the ICU (20.5 [range, 5 to 41] vs 18 [range, 4 to 40], respectively; p = 0.6) and Charlson comorbidity index score (2 [range, 0 to 6] vs 2 [range, 0 to 6], respectively; p = 0.9). The median time to the start of appropriate treatment was 68.5 h (range, 45 to 149 h) in the delayed-treatment group and 25 h (range, 4 to 44 h) in the early-treatment group. Only 16 patients received appropriate antibiotic treatment within 24 h of the onset of infection.
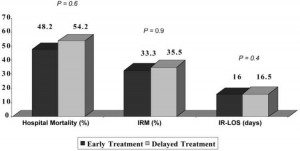
 No differences existed with respect to the primary outcomes based on the receipt of delayed vs early appropriate antibiotic therapy (Fig 4). In addition, no significant difference in the rate of response was seen at days 3, 7, and 10, and at the end of antimicrobial therapy or the first day to clinical improvement (5 days [range, 2 to 14 days] vs 5 days [range, 2 to 11 days], respectively). In the CART analysis that was performed to identify a specific “time to therapy” for the bacteremic S aureus pneumonia cohort, no additional time breakpoint in appropriate antibiotic treatment was found that produced an increased probability of IRM.
No differences existed with respect to the primary outcomes based on the receipt of delayed vs early appropriate antibiotic therapy (Fig 4). In addition, no significant difference in the rate of response was seen at days 3, 7, and 10, and at the end of antimicrobial therapy or the first day to clinical improvement (5 days [range, 2 to 14 days] vs 5 days [range, 2 to 11 days], respectively). In the CART analysis that was performed to identify a specific “time to therapy” for the bacteremic S aureus pneumonia cohort, no additional time breakpoint in appropriate antibiotic treatment was found that produced an increased probability of IRM.
The evaluation of the appropriateness of therapy for concomitant pathogens was also assessed. Alternative pathogens were appropriately treated 85% of the time based on the susceptibility profile. The clinical success was 59.5% for patients treated appropriately for both NBSAP and the alternative pathogen. This result was similar to the clinical success rate observed for the entire cohort (56.7%).
Complete, infection-related cost data were available for 22 of the 60 patients. The greatest reason for increased cost was length of hospitalization. In the patients who lived, the median total cost was $35,072 (range, $19,764 to $312,511) compared with $22,098 (range, $1,218 to $66,351) in the patients who died. No differences in cost were evident in patients based on methicillin susceptibility or early empiric treatment.
Figure 1. IRM grouped by APACHE II score. APACHE II scores grouped in values of 10. Dotted line represented linear regression line.
Figure 2. Receipt of adequate treatment within the 44.75-h breakpoint established for S aureus bacteremia.
Figure 3. Outcomes of NBSAP based on MSSA vs MRSA pneumonia.
Figure 4. Outcomes of NBSAP based on early vs delayed treatment.
Table 1—Characteristics of MSSA vs MRSA Pneumonia
| Characteristics | MSSA (n = 18) | MRSA (n = 42) | p Value |
| Age, yr | 55.2 ± 17.6 | 58.4 ± 13.9 | 0.45 |
| APACHE II score | 20.5 (5-41) | 20.0 (3-36) | 0.72 |
| Charlson index score | 2 (0–6) | 2(0-7) | 0.53 |
| Diabetes | 3(16.7) | 15 (35.7) | 0.14 |
| Decubitus ulcers | 0 | 7(16.7) | 0.09 |
| Prior antibiotics | 3(16.7) | 30(71.4) | < 0.01 |
| Vent at NBSAP onset | 13(72.2) | 37 (88.1) | 0.13 |
| LOS prior to NBSAP onset, d | 4 (2-31) | 11.5 (4-81) | 0.03 |
| ICU LOS prior to NBSAP onset, d | 3(0-31) | 9(0-58) | 0.059 |
| Time to adequate therapy, h | 24(8-51) | 44(1-149) | 0.013 |